Healthy Skepticism AdWatch
AdWatch illuminates the logical, psychological and pharmacological techniques used in drug advertisements.
March 2007, Australia
Avandia (rosiglitazone) from GlaxoSmithKline
Do not ADOPT rosiglitazone as first-line diabetes treatment.
This advertisement for Avandia (rosiglitazone) published in Medicine Today, volume 8(1), January 2007, is based on the results of the ADOPT (A Diabetes Outcome Progression Trial) trial, recently published in the New England Journal of Medicine (2006; 355; 23-2427-43).

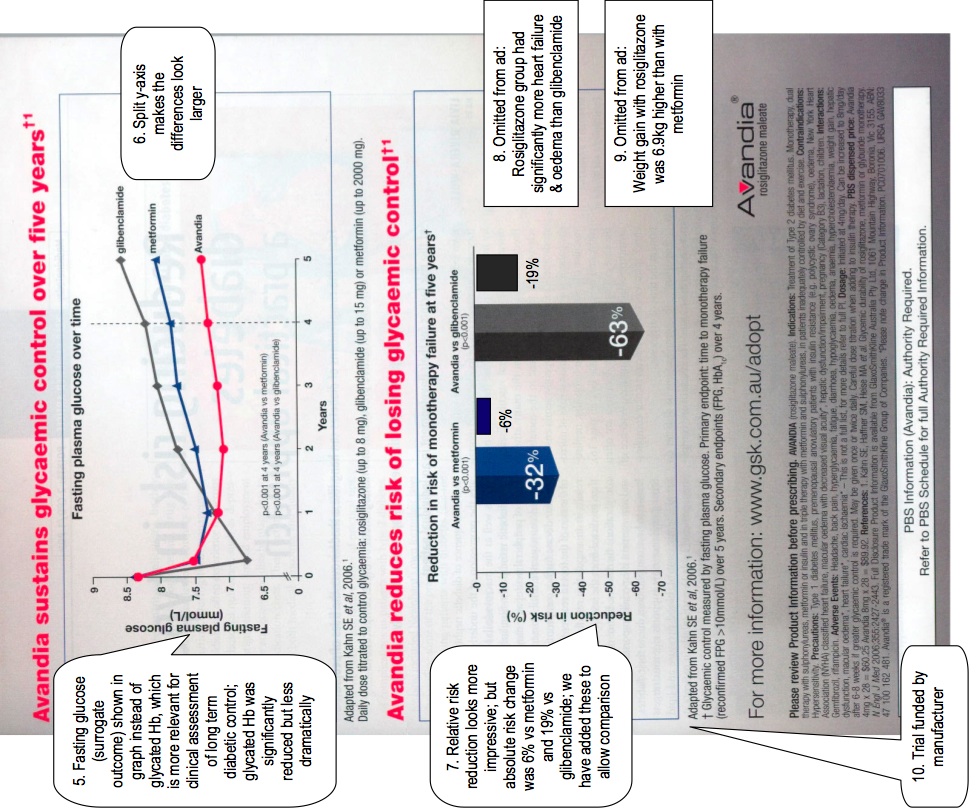
The AdWatch team are concerned that the advertisement uses several techniques that exaggerate the benefits and minimize the harms associated with the drug.
1. The name of the trial is an unjustified promotional verb.
2. Early studies with rosiglitazone suggested that it was no more effective in lowering glycaemia than were older oral antidiabetic medications. Unlike metformin and sulphonylureas in the UKPDS (United Kingdom Prospective Diabetes Study) trial, Avandia has NEVER been shown to reduce any diabetes-related complication in long-term clinical trials. Avandia causes significant adverse effects (including weight gain, fluid retention and heart failure). So far, the Pharmaceutical Benefits Advisory Committee (the Australian Government committee that recommends whether drugs should be subsidized) has not recommended it for first-line treatment.
3. In Australia, rosiglitazone is subsidized only for ‘add-on’ therapy for people already on other anti-diabetic drugs, and not for monotherapy. The ADOPT trial only tested monotherapy. Extrapolation from ADOPT to ‘add-on’ therapy may not be justified.
4. Only about 60% of the cohort completed the study. The loss of so many patients may have distorted the results.
The results of the trial are expressed in two graphs in the advertisement.
5. The line graph shows glycaemic control in terms of fasting blood glucose, although a more stable indicator of control, that is used clinically to indicate long term control, is glycated Hb. The glycated Hb was significantly better in the rosiglitazone group as well, but the difference was less dramatic (0.13% [95% confidence interval 0.22-0.05] lower than metformin , 0.42% [0.50, 0.33] lower than glibenclamide). The glycated Hb is plotted below with a split y-axis similar to the split y-axis used in the advertisement:
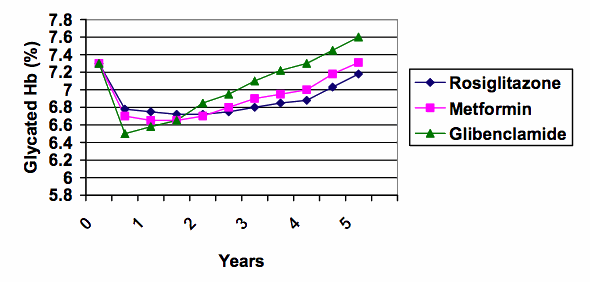
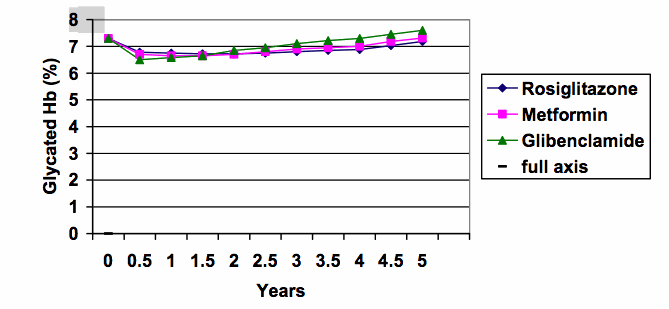
6. Split y-axes can give an exaggerated impression. When the same data are graphed using the full scale from zero (see below), the differences between the treatment groups are put into much better perspective (the range of results at 5 years was about 7.2 to 7.6%).
7. In the bar graph, relative risk reduction has been used instead of absolute risk reduction, again with the effect of exaggerating the effects. The incidence of monotherapy failure in each group was:
• Glibenclamide 34%
• Metformin 21%
• Rosiglitazone 15% absolute difference from M = 6%; = 32% x 21% (relative risk)
absolute difference from G = 19%; = 62% x 34% (relative risk)
The absolute risk reduction is much more informative but looks less impressive.
8. There was a statistically significantly higher risk of cardiac failure in the rosiglitazone group (absolute risk 1.5%) compared with glibenclamide (absolute risk 0.6%). This occurred in a group of patients selected NOT to have pre-existing heart failure. This information is not included in the advertisement at all, despite being one of the important findings in the trial. We are not aware of any study of how dangerous rosiglitazone is for diabetics with pre-existing heart failure.
9. The weight gain in the rosiglitazone group was 6.9 kg [95% CI 6.3, 7.4] higher than in the metformin group and 2.5 kg [2.0, 3.3] higher than in the glibenclamide group.
10. The ADOPT study was funded by GlaxoSmithKline. Sponsor-funded trials are much more likely to report positive results than independently funded trials.[1]
Late news: in February 2007, GlaxoSmithKline has sent a warning letter to health professionals informing them of an increased incidence of fractures in female patients taking Avandia compared with those taking metformin or glibenclamide in the ADOPT trial. While these results need to be confirmed in other studies, this is a reminder that it is not wise to use new drugs with unknown long-term efficacy and safety, while other better evaluated treatments are available.
The AdWatch team concludes that this advertisement is based on a questionable extrapolation. It also exaggerates the benefits and minimizes the harms associated with the drug.
1. Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Can Med Assoc J. 2004;170(4):477-80.
Indexes:
AdWatch (Australia)
Page views since 15 March 2010: 10172
Comments
Our members can see and make comments on this page.


