Healthy Skepticism International News
May 2009
Proposal for an internet database of phamaceutical advertisements
There should be an internet database of pharmaceutical advertisements
Staffan Svensson
Angered Health Care Centre and Dept of Clinical Pharmacology, University of Gothenburg, Sweden
1 Background
Pharmaceutical advertising is contentious in that it is sometimes used to promote treatments that are unnecessarily expensive, not needed or even harmful. Reactions of consumers1 to pharma ads are important for raising debate and alerting government agencies to the problem – actions that may lessen the impact of dubious ad campaigns and lead to better promotion in the future.
However, while drug advertising campaigns are rolled out globally, response to them is mostly local. Consumers in one country are therefore often unaware of what goes on elsewhere, so the whole intellectual process of assessing the ads has to be started anew in each place. A system for making information about ads easily available could reduce such duplication of efforts and facilitate ad surveillance.
2 Suggestion
A relational database of pharmaceutical advertisements should be created and made available on the internet for public access and input. It could be developed and hosted by Health Action International or some other NGO with an interest in consumer protection and pharmaceuticals. The database would need the oversight of moderators, who could be recruited through the NGO.
3 Contents
Input data would be in two categories: factual and interpretative. The factual content would be the ad itself as an image or video file (or link), and associated info about it. The interpretative content would be comments and assessments concerning the ads. As few things as possible should be compulsory, in order to maximise input.
3.1 Possible variables
1. Factual
- The ad file or link to it
- Generic name
- Trade (brand) name
- Company
- ATC code
- Diagnosis promoted for
- Text of ad
- References used
- Language
- Translation language
- Date of publication
- Parent campaign
- Start of campaign
- End of campaign
- Geographical scope of campaign
- OTC or prescription
- Source type
- Data type (still/moving)
- Date of submission
- Contested (reported to authority)
- Contested in lawsuit
- Entered by
[1]Ad consumers are those to whom the ads are directed – prescribing health care professionals or patients.
2. Interpretative
Punchline/main message
- Type of message – likely mechanism
- Assessment of factual message
- Assessment of reference adequacy
- Interpretation of visual imagery
- Assessment of emotional message
- Translation in English
- Translation in other languages
- Outcomes of any assessment board review
- Outcomes of any lawsuits
- Relations to other ads
- Press coverage
- References to debate, links
- Various comments
3.2 Editing
Members of the public should be able to add information to the database. Minimal registration procedures (basically proof of humanity) should apply. Addition of new categories by users should be possible, such as diagnoses and companies. Some categories could be predefined, eg ATC codes and countries. It should be possible for users to define and save different views of the data,
for future use and for referencing. Unlike a true wiki database, editing and deletion of existing information should be possible for selected (registered and approved) users and moderators only.
Users should be able to export search results to common formats, as well as download the entire database.
4 A simulated example
Let’s say an ad campaign for allergy awareness is launched in Spain. No drugs are explicitly named, but a pharma company’s name (Schering-Plough) is in the ad. Someone in Spain wants to know more about it and enters “allergy” in the database, and at the same time modifies the display to show more variables – diagnosis, ATC code and country (Figure 1).
This yields four instances of ads by this company: two for an antihistamine (desloratadine) and two for a nasal steroid (Figure 2).
Looking at the first ad, from France, the user recognises the design and vicious purple colour in the Spanish ad, thereby confirming it is part of a campaign (Figure 3). The message is similar between these two manifestations of the campaign.
Looking at the next instance, from Sweden, some more data is available: this ad had more extensive text as well as a self-test. Also, the ad has been reported to a review board for alleged misleading claims (Figure 4).
The Spanish user now has an idea of the history of the ad, as well as some of the reactions it has spawned in other countries. Possible next steps would be to create a new entry by uploading an image of the Spanish ad with details, and perhaps trying to get hold of the results of the Swedish authority’s assessment.
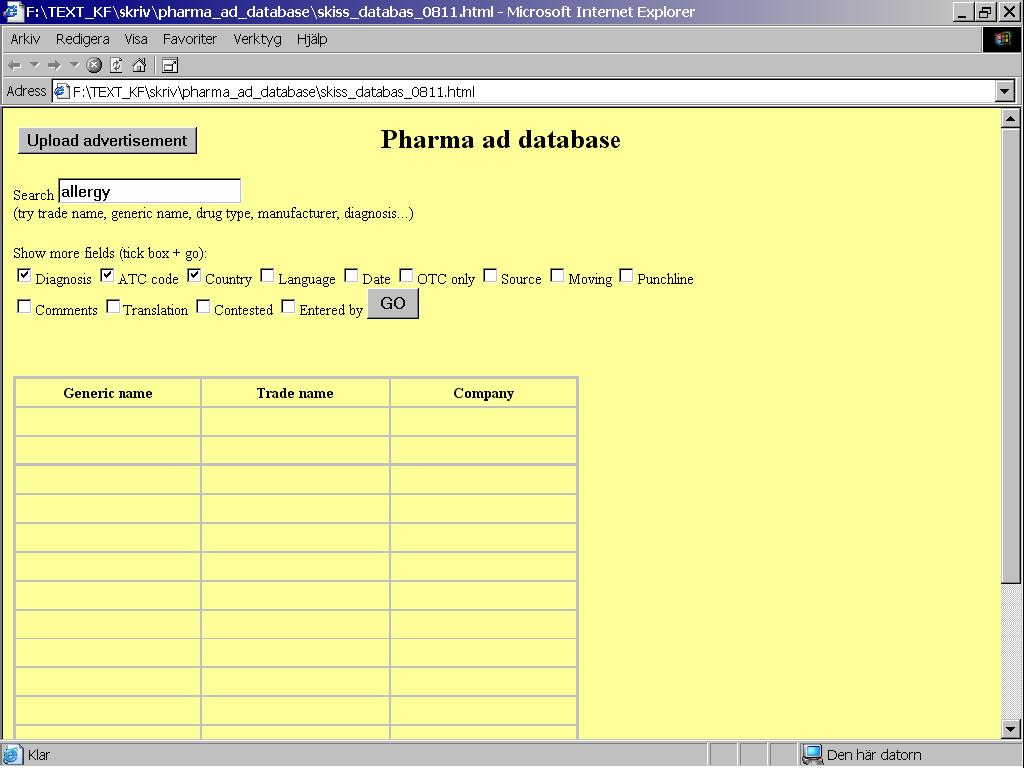
Figure 1: A simulated search
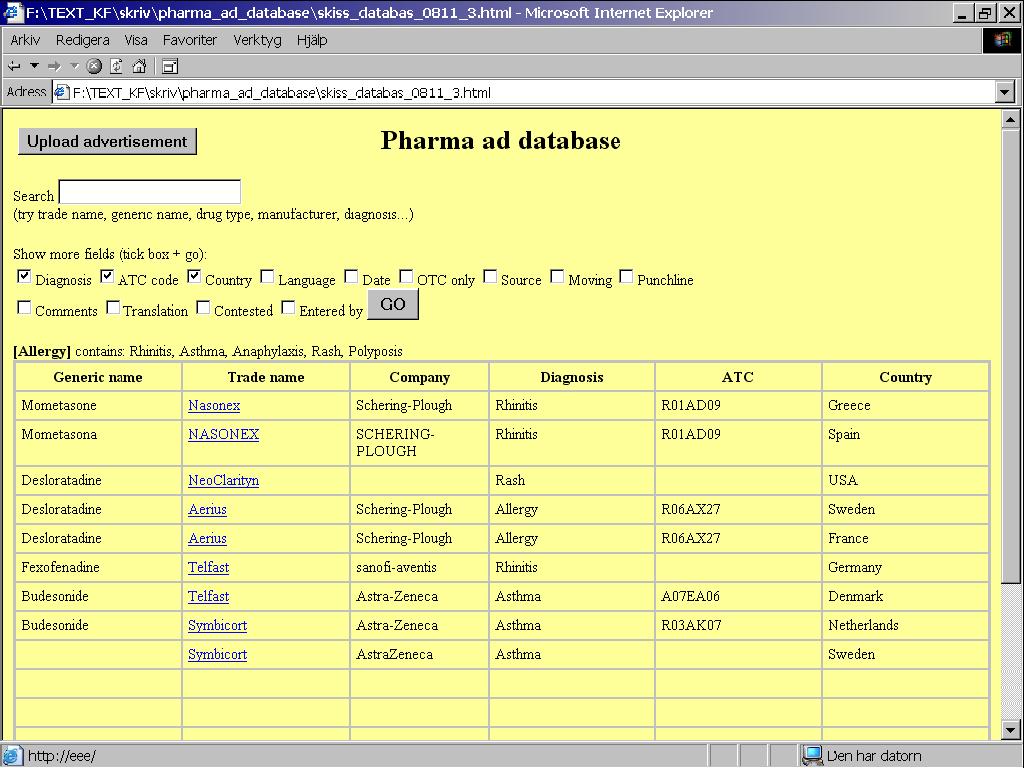
Figure 2: Search results
5 Potential problems
- Lack of interest
- Lack of funding for programming and web space
- No moderators
- Computational demands too complex
- Chaotic results of user creation of categories
- Vandalism, spamming, robot entries, fakes
- Hi-jacking by fanatics such as scientologists
- Copyright infringement of ads
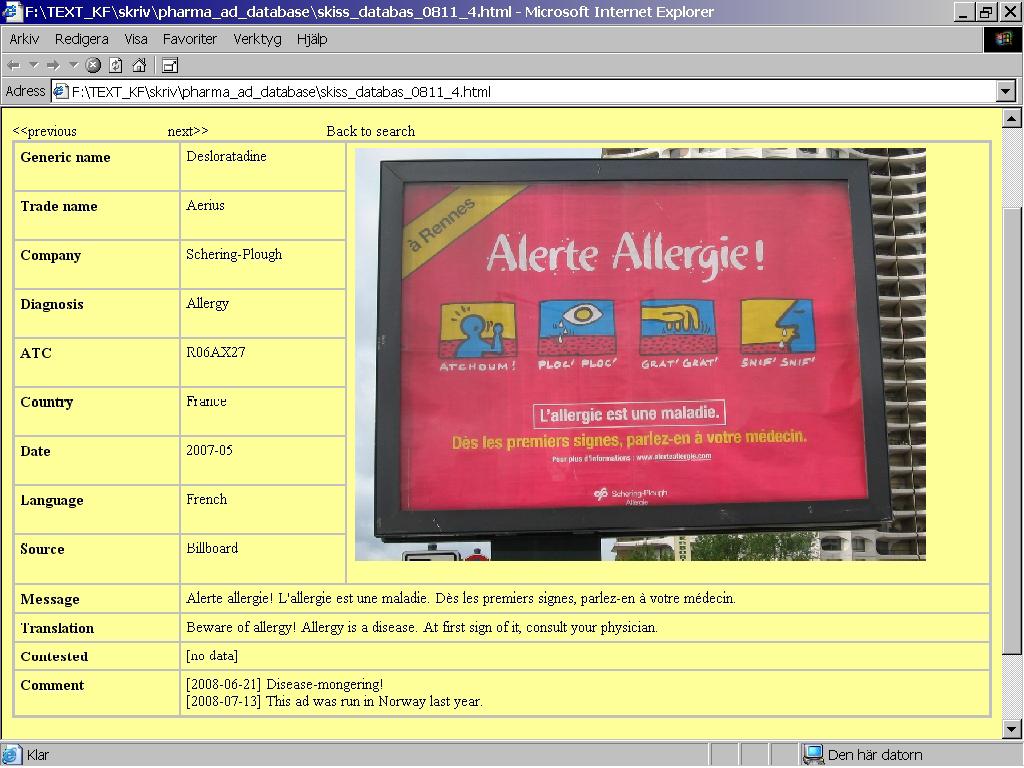
Figure 3: French database entry
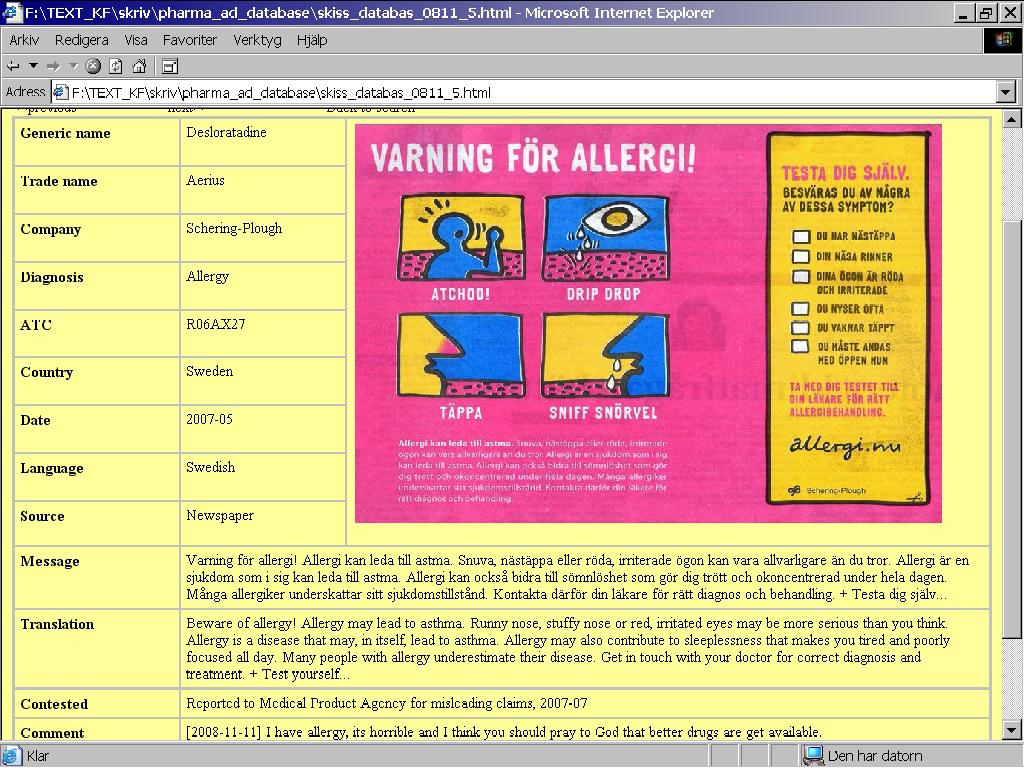
Figure 4: Swedish database entry
6 Potential benefits
- Help consumers find information about ads
- Provide a forum for discussion about ads
- Make co-ordinated activities easier
- Allow tracking of campaign messages’ development over time, in response to reactions
- Follow assessments of authorities and legal bodies in various countries
- Facilitate research
Page views since 15 March 2010: 5688
Comments
Our members can see and make comments on this page.
















