Healthy Skepticism International News
Healthy Scepticism about Pfizer and Searle’s promotion of celecoxib in Australia
March 2000
Peter Mansfield
Email : .(JavaScript must be enabled to view this email address)
Introduction
A copy of the advertisement for Celebrex (celecoxib) published in Australia in Current Therapeutics Dec 1999 / Jan 2000, vol 40, no 12 appears below.
Celecoxib is a NSAID (New Sort of Aspirin In Disguise). It may turn out to be a useful alternative for people with osteoarthritis or rheumatoid arthritis. However the promotion of Celebrex shows that little is new under the sun or the moon.
In Mabeth, Hecate (the leader of the witches) describes her plans to use a drug to create sprites (apparitions) to fool Macbeth. The sprites use ambiguous claims (more than one meaning each) that lull Macbeth into a false sense of security because he hears only the meaning that he wants to hear. Macbeth’s illusion of security is that he can act decisively to fulfil his hopes without fear of adverse effects.
Hecate: …Upon the corner of the moon
There hangs a vaporous drop profound;
I’ll catch it ere it come to ground:
And that distill’d by magic sleights
Shall raise such artificial sprites
As by the strength of their illusion
Shall draw him on to his confusion:
He shall spurn fate, scorn death and bear
His hopes ‘bove wisdom grace and fear;
And you all know security
Is mortals’ chiefest enemy.
- Macbeth Act III Scene 5
 |
 |
The headlines
Advertisers know that many people only scan the headlines and pictures without reading the smaller print copy let alone the fine print. Consequently the name of the product and main messages (always benefits only) go in large type.
“Potentially the most exciting therapeutic advance in the treatment of inflammation and pain since the advent of NSAIDs.”
“Potentially” is a weasel word. It is a word that enables the companies to evade responsibility if the rest of the sentence conveys an impression that turns out to be false. If so then Pfizer and Searle are still safe. What about you and your patients?
“the most exciting therapeutic advance”
Who would want to miss out on the good feelings of excitement, enthusiasm and fulfilment of hope to be gained from uncritical faith?
“the treatment of inflammation and pain”
Drug companies often claim that they only promote their products for the indications approved by the Therapeutic Goods Administration (TGA). However the TGA have approved celecoxib for symptomatic treatment of osteoarthritis and rheumatoid arthritis only. “Inflammation and pain” arise from many other causes for which celecoxib has not been approved because of lack of evidence. The headline appears to be designed to break the rules and expand the market so Pfizer and Searle can make more money. It is true that the approved indications are mentioned in smaller print but many people do not read the smaller print.
“since the advent of NSAIDs.”
It appears that Pfizer and Searle would like us to compare celecoxib with NSAIDs only and forget about other alternatives that will be much better for many patients including paracetamol, physical therapy, injury prevention and disease modifying drugs.
“Prove it!”
This gives the impression that proof is provided in the copy so that people who do not read the copy can feel reassured. However the main aim of this headline appears to be to get the eye to the start of the copy and to motivate the reader to read the copy.
“New”
The word new is used because controlled trials of advertising have shown that placing it prominently in an advertisement increases sales. It is normal for doctors to want to “keep up” with new therapies. The advertisement is designed to take advantage of that motivation. It is a good thing if the therapy turns out to be better than established therapies. However new drugs are almost always less well tested in “real world” conditions and are almost always more expensive for patients and/or taxpayers. It is wise to remember the example of Posicor (mibefradil) which was launched on the Australian market with great enthusiasm but was withdrawn internationally a week later because of problems with drug interactions. Switching quickly to new drugs is taking a risk with your patients’ health. You may be right but you may be wrong!
“Cerebrex”
Trade names are chosen to take advantage of conscious or unconscious connotations. In this case the connotations include “celebrate”. That association is reinforced by a picture of a small person who appears to be celebrating by jumping (as in the TV adverts for Toyota) to form an X which in turn is reinforced with the x at the end of the Trade name.
“Powerful relief, safely delivered”
These 4 carefully chosen words are open to a range of meanings. If you thought they mean that celecoxib is “Reliably effective with few or no adverse effects” then you have been mislead. A more correct statement would be “No more effective than NSAIDs (which often disappoint) with long term adverse effect profile in general use unknown.” (More about the adverse effects of celecoxib later.)
The four words in the slogan all have emotive connotations that appeal consciously or unconsciously to some of the finer motivations of health professionals. These include the desires to be powerful for good, to relieve suffering and to deliver safe care. Can you resist these worthy appeals? Many doctors believe that such appeals have little influence. However evidence from marketing research shows that repetition of associations between a drug and a desirable image will make that drug the first thing that you think of when deciding what to prescribe. Unfortunately little evidence is available yet on whether or not Cerebrex will deliver on the promises implied in the hype. The promises are merely implied but not clearly spelt out so that Pfizer and Searle will be safe if unexpected problems occur. Will your patients be safe? No one knows.
The copy
The copy in this advertisement is a major improvement on that in earlier advertisements for Celebrex that focused on mechanisms. Knowledge of mechanisms is not the key to deciding whether or not to use a new drug. The key issue is: Is there good evidence that the new drug has better ratios of efficacy, adverse effects and costs than current therapy?
1 “The first COX-2 Specific Inhibitor (CSI), Celebrex (celecoxib), is a new way to treat the pain and inflammation of osteo- and rheumatoid arthritis.” If the claim that celecoxib is a “Specific Inhibitor” suggests an absolute lack of effect on COX-1 then such a strong claim is not justified. The possibility that celecoxib has a small but clinically important impact on COX-1 in some people, at some doses over time has not been excluded. Meanwhile it would be justified to claim that celecoxib has less effect on COX-1 than other NSAIDs.
2 “This new class of drugs is likely to completely rewrite prescribing habits in the next few years.” Pfizer and Searle want to make a lot of money. They do not want use of celecoxib limited only to patients at high risk of ulcers.
3 “Has the hard work been done which will allow you to feel confident about efficacy and more importantly, tolerability?”Please do not think about the cost for the patient of this overpriced drug! By contrast, Pfizer and Searle are concerned about making doctors feel confident. It is important for sales. Shakespeare also understood the importance of confidence. He described a false sense of security as “mortals’ chiefest enemy” in Macbeth.
4 “There have been more than 50 trials of Celebrex in 23 countries including Australia.[1]” Reference one is “Data on file”. Very few of the 50 trials have been published so only Pfizer and Searle’s side of the story is available for most of the evidence. However, to be fair to Pfizer and Searle there are a few trials that could not be cited when the advertisement was written but have been published since then.
5 “Over 13,000 patients have been studied.[1]” “Data on file” again. This sounds impressive but how many of the 13,000 have been high risk patients taking the drug long term (5-10 years) ? This is important because people often take treatments for osteoarthritis or rheumatoid arthritis for decades.
6 “Approximately 2,300 patients have received Celebrex for a year or more.[1]” Therefore we can forget about 10,700 of the 13,000. One year was not long enough to detect unexpected problems with many other drugs including NSAIDs.
7 “Gastric safety has been studied with 10,500 endoscopies in over 4,700 patients.1” “Data on file” again. Either Pfizer and Searle are double dipping in the data or all of the 10,500 took celecoxib for less than a year. Even if they are double dipping, the majority took celecoxib for less than a year.
8 “Celebrex has also been studied in over 900 patients also taking methotrexate, DMARSs or steroids.[1],[3]” But for how long? “Data on file” again. Reference 3 was published in a supplement. There is evidence that studies published in supplements (which bypass the usual editorial quality control systems) may be of lower standard than studies published properly. The advertisement provides no information about the results of those studies. Why not?
9 The next 7 sentences are essentially more of the same. Feel free to skip them and go direct to sentence 18 or the conclusion (sentence 19).
10 “In large, randomised, double-blind, placebo- and active controlled trials Celebrex relieved the pain and inflammation of osteoarthritis as effectively as high dose naproxen (1000mg daily) and diclofenac 150mg daily).[1],[2]” “Data on file” again. Reference 2 is the Product Information. It does not provide enough information to enable evaluation of the quality of the trials. How large is “large”? Were the trails large enough? What assumptions were used for the power calculations? Was there adequate concealment of allocation (a key test of the quality of trials) ? What dose of celecoxib was used? Is celecoxib superior to paracetamol by a clinically important difference? If this has not been tested why not? Was it ethical to use a placebo? (None of these questions are just rhetorical. I do not know the answers but need to know before I can make a proper assessment of this drug.)
11 “And in rheumatoid arthritis, Celebrex was as effective as high dose diclofenac (150mg) in reducing the number of swollen and painful joints [1],[4]” “Data on file” again. Reference 4 is another supplement. Did the patients feel that the difference was clinically important or not? What dose of celecoxib was used? What about other measures of efficacy?
12 “It was also effective in improving functional ability in both OA and RA.[1]”“Data on file” again. No other references. No other information. Does this mean that no improvements in functional ability were found in references 3 and 4.
13 “However, the really dramatic difference was in terms of safety.” “Safety” has very reassuring connotations that we all want to hear but that emotive word may mislead. Very few effective drugs are “safe”.
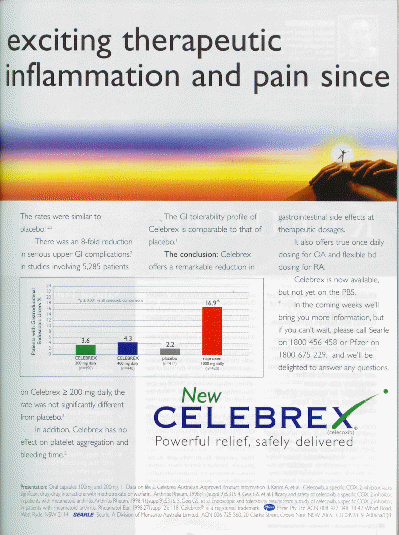
14 “In the trials conducted, Celebrex was associated with significantly fewer GI ulcers than naproxen, diclofenac or ibuprofen. The rates were similar to placebo.[1],[2],[5]” “Data on file” again. Product information again. Reference 5 is yet another supplement. These results are illustrated by a graph that does not disclose the 95% confidence limits. We are not told the duration of the study. That is important because the frequency of ulcers may increase with time with the drugs but not so much in the placebo group. The difference between naproxen and celecoxib certainly looks like it could be clinically important. The absolute risk difference of 2.1% between celecoxib and naproxen may also be clinically important because just one percent is a lot of people if the drug becomes widely used. The lack of statistical significance could be a type 2 error because the number of people in the study is so small. In relative risk terms the difference is an additional 95% more endoscopic ulcers with celecoxib than with placebo (almost a doubling of risk). In relative terms this dwarfs the differences made by so-called “powerful” cholesterol lowering drugs which reduce relative risks by around 30%. Endoscopic ulcers are a surrogate endpoint. The relationship between endoscopic ulcers and clinically important events is not clear cut. Ulcers associated with celecoxib may or may not go on to clinically important problems at a much higher rate than with placebo. Consequently the next sentence is more important.
15 “There was an 8-fold reduction in serious upper GI complications.[2]” The product information again. No information on numbers, duration, statistical significance etc. Why tell us so little about a study that is so important?
16 “In studies involving 5,285 patients on Celebrex > 200mg daily, the rate was not significantly different from placebo.[2]” The product information again. That sentence does provide information about the numbers and statistical significance but not about duration.
17 “In addition, Celebrex has no effect on platelet aggregation and bleeding time.[2]” Product information again. That is a very strong absolute claim. It may be true of clinical trials so far. I hope it will also be true when the drug is taken by a wider range of people with all sorts of other health problems.
18 “The GI tolerability profile of Celebrex is comparable to that of placebo.” This is ambiguous. The term “comparable to” could be taken to mean “the same as” or “almost the same as” but it could also mean that you can compare the two rates and find that there is a clinically important difference.
19 “The conclusion: Celebrex offers a remarkable reduction in gastrointestinal side effects at therapeutic doses.” An alternative conclusion is: One small study has found a statistically significant lower rate of endoscopic ulcers with celecoxib 200mg daily and 400mg daily compared to naproxen 1000mg. There was a trend (that may be dose related) towards more endoscopic ulcers with celecoxib compared to placebo. Whether or not there are any clinically important differences in the rates of clinically important adverse events between placebo, celecoxib and other NSAIDs is not yet known.
Comments
Our members can see and make comments on this page.
















