Healthy Skepticism AdWatch
AdWatch illuminates the logical, psychological and pharmacological techniques used in drug advertisements.
October 2009, USA
Amylin and Eli Lilly’s Byetta® (exenatide injection) for type 2 diabetes
This advertisement is promoting a drug for type 2 diabetes on the basis of a surrogate outcome assessed in unpublished trials, and as an off-label treatment for overweight and obesity.
This advertisement appeared in the August 20, 2008 issue of Journal of the American Medical Association and elsewhere.
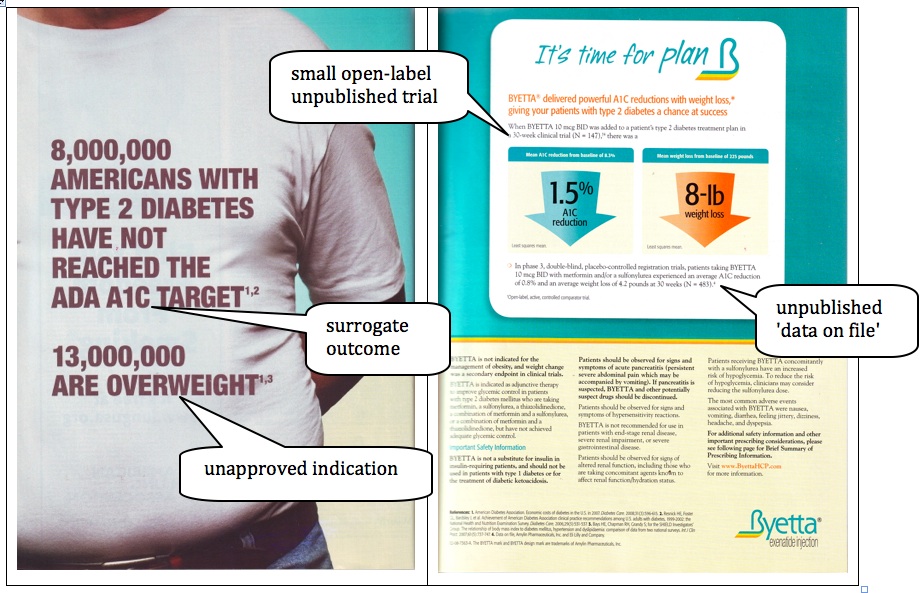
Headline
8,000,000 AMERICANS WITH TYPE 2 DIABETES HAVE NOT REACHED THE ADA A1C TARGET
13,000,000 ARE OVERWEIGHT
It’s time for plan ß
The main headline misleads by focusing on haemoglobin A1c, a surrogate outcome measure that is widely believed to be a good predictor of important outcomes such as heart attacks and strokes but is actually not (for example, see McCormack and Greenhalgh’s critical analysis of interpretations of UKPDS findings).[1] Recent warnings about the use of rosiglitazone, an anti-diabetic drug that was widely promoted for its effects on haemoglobin A1c, but was then shown to have detrimental cardiovascular effects,[2] are a good reminder of the importance of not relying on surrogate outcomes for the assessment of new drugs,[3] in particular anti-diabetic drugs.
The headline also may mislead by indirectly promoting use of Byetta for weight loss in addition to glycemic control. The statement that 13 million are overweight could be misinterpreted to mean that there are 13 million Americans generally (not 13 million diabetic Americans) who are overweight, and that Byetta can be used for weight loss even by people who are not diabetic. At worst, this may indicate off-label promotion for an unapproved indication. In fact, less than a year after Byetta was introduced to the US market in June 2005, it was being used by people who were not diabetic, and this was being subtly promoted in the media, for example in the New York Times.[4] This off-label promotion could be helped further by a recent news release about a small study presented at the annual meeting of the US Endocrine Society claiming that ‘Byetta may aid weight loss in obese patients’.[5] At best, the emphasis on Byetta’s weight loss effect is misleading because it is presented as a significant advantage compared with available treatments. The Australian Pharmaceutical Benefits Advisory Committee, the advisory body that advises the Australian Health Minister about which drugs should be subsidised by the Pharmaceutical Benefits Scheme, rejected the weight loss claim and stated that ‘the long term benefits and durability of weight change with exenatide have not been adequately established’.[6] It also stated that ‘This benefit has not been verified in a properly designed weight loss study or quality of life studies, nor has it been shown to be durable over the long term’ and it has ‘not been shown to result in improved morbidity and mortality outcomes’.[7]
Brand name
Possible connotations of the name Byetta include ‘Beta cells’ (diabetes occurs when the beta cells in the pancreas do not make enough insulin) and ‘Better’. Another possible connotation is ‘Bye eating’, which may reinforce the promotion of use of Byetta for weight loss discussed above.
Logo
The logo for the drug is the word Byetta with a distinctive B in a shape similar to the Greek letter beta (ß), reinforcing the association with beta cells.
Slogan
The slogan, which is the headline on the second page, is: ‘It’s time for plan B’. The B in plan B is written in the same way as the first letter of the drug name, so the slogan is effectively: ‘It’s time for plan Byetta’.
Picture
The picture is of the chest and abdomen of an obese man. This reinforces the promotion of Byetta for people who are overweight.
Copy
The first sentence of the copy is in large print and states: ‘BYETTA® delivered powerful A1C reductions with weight loss,* giving your patients with type 2 diabetes a chance at success’. This reinforces the misleading messages in the headline. The asterisk (*) links this sentence to a fine print disclaimer that partly contradicts the message: ‘*BYETTA is not indicated for the management of obesity, and weight change was a secondary endpoint in clinical trials’. The use of fine print disclaimers does not stop a larger print statement from being potentially misleading for the majority of people who do not read the fine print.
The copy mentions in fine print the results of two unpublished ‘data on file’ clinical trials. For the first small trial (n=147), there is a fine print disclaimer revealing that it was an ‘Open-label, active, controlled comparator trial’, i.e. not a good enough quality trial to produce reliable results. More information is given about the second trial, which is of higher quality. The results for haemoglobin A1c and weight from the first trial were almost twice as good as those from the second trial.
The first trial could be misleading because it was lower quality and/or because other things were done to produce the better results. Only the results of this trial are presented in large type highlighted with arrows (blue and orange, echoing the colours of the Byetta logo). It is disclosed in fine print that the results are least squares means. Few readers would know the difference between ordinary means and least squared means. Because we have limited information about the trial design and results, we are unable to judge the appropriateness of using least squares means rather than ordinary means. Unless the results for all participants in the trial are very similar, any type of mean can be misleading.
References
Three of the four references provide information about diabetes but not about Byetta. The fourth is data on file, i.e. information that has not been subjected to peer review.
Fine print
There is one third of a page of small but readable fine print on page 2, then one page of very fine print (not shown) that is difficult to read. Evaluation of the details of the very fine print is beyond the scope of this AdWatch.
What’s missing?
The advertisement does not provide comparisons with recommended alternative treatments, or information about effects on important outcomes such as deaths, heart attacks and strokes. The headlines, picture and copy provide no information about adverse effects or warnings. Without that information, it is not possible to make a good decision regarding use of this drug. The advertisement also does not provide any information about cost.
Overall
The headline, picture and copy emphasise an unapproved indication (overweight) and a surrogate outcome measure (haemoglobin A1c) with an uncertain effect on important endpoints. This distracts attention from more important outcomes such as deaths, heart attacks and strokes.
A superficial reading will mislead many people to the impression that the drug is a first choice for people with type 2 diabetes who are overweight (the majority), rather than an add-on treatment. The advertisement only includes a selection of the evidence. It does not include any data from trials with relevant comparators such as insulin. In fact, the advertisement does not make reference to the results of other studies comparing exenatide with insulin glargine or insulin aspart which showed that exenatide had no superior glucose-lowering effect compared to these insulins.[8]
Furthermore, the advertisement does not include any warning about the fact that, like all new drugs, Byetta could cause severe adverse effects that might be uncovered when the drug is used in the wider population. In fact, there are worrying reports of Byetta-associated renal failure[9] and pancreatitis that may offset any potential benefits associated with weight loss.
About 80 per cent of people do not give advertisements more than a superficial inspection.[10] Busy health professionals who do not spend a lot of time examining this advertisement are likely to be misled.
IFPMA Code breaches
This advertisement breaches the requirement of section 3 of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) Code that: ‘No pharmaceutical product shall be promoted for use in a specific country until the requisite approval for marketing for such use has been given in that country’. It does this by promoting the use of Byetta for overweight in the headline, picture and copy.
This advertisement breaches section 4.2 of the IFPMA Code in several ways including:
• The sections of the advertisement that are most likely to be read are not balanced: The headline, picture and copy do not provide any information about adverse effects, warnings, prices etc.
• The advertisement is not sufficiently complete to enable the recipient to form his or her own opinion of the therapeutic value of the pharmaceutical product concerned: The advertisement does not provide any comparative information regarding efficacy, adverse effects or prices.
• The advertisement is misleading in several ways mentioned above, including misleading via omission of information about adverse events in the trials and misleads by placing undue emphasis on the more favourable looking results from a lower quality trial over the less favourable looking results from a better quality trial. It also misleads by promoting an unapproved indication (overweight).
This advertisement breaches the requirement of section 5.1 of the IFPMA code that the date of production of the advertisement be stated. There is a code number which may contain date information but that is not clear.
1. McCormack J, Greenhalgh T. Seeing what you want to see in randomised controlled trials: versions and perversions of UKPDS data. United Kingdom prospective diabetes study. BMJ. 2000 Jun 24;320(7251):1720-3.
2. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007 Jun 14;356(24):2457-71. Epub 2007 May 21 [cited 1 July 2009]. Available from: http://content.nejm.org/cgi/content/full/356/24/2457.
3. Twaddell S. Surrogate outcome markers in research and clinical practice. Aust Prescr. 2009 Apr;32(2):47-50 [cited 23 September 2009]. Available from: http://www.australianprescriber.com/magazine/32/2/47/50.
4. Berenson A. A ray of hope for diabetics. New York Times. 2006 Mar 2 [cited 25 June 2009]. Available from: http://www.nytimes.com/2006/03/02/business/02drug.html.
5. Thomas J. Diabetes drug Byetta may aid weight loss in obese patients. HealthDay. 2009 Jun 11 [cited 25 June 2009]. Available from: http://www.healthday.com/Article.asp?AID=628005.
6. Pharmaceutical Benefits Advisory Committee 2008, Public Summary Document for Byetta November 2008 [cited 26 June 2009], Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/A51E05907F7B6ED2CA25756A0002A83E/$File/pbac-psd-exenatide-nov08.pdf.
7. Pharmaceutical Benefits Advisory Committee 2007, Public Summary Document for Byetta July 2007 [cited 26 June 2009]. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/A20245908EC3CCE5CA2573860011C075/$File/Exenatide%20Byetta%20PSD%20Eli%20Lilly%20FINALv2.pdf.
8. Prescrire. Exenatide: Type 2 diabetes for some overweight patients. Prescrire International. 2007 Dec;18(101):228-31.
9. Prescrire. Exenatide: renal failure. Prescrire International. 2009 Jun;18(101):124.
10. Ogilvy D. Ogilvy on advertising. London: Prion; 1995.
Indexes:
AdWatch (USA)
Page views since 15 March 2010: 21038
Comments
Our members can see and make comments on this page.


