Healthy Skepticism AdWatch
AdWatch illuminates the logical, psychological and pharmacological techniques used in drug advertisements.
June 2007, Australia
Celebrex (celecoxib) from Pfizer
Is consumer health information from drug companies trustworthy? This AdWatch analyses an advertorial by Pfizer published in the Australian edition of the Readers Digest in March 2007.
This AdWatch analyses an advertorial by Pfizer published in the Australian edition of the Readers Digest in March 2007.
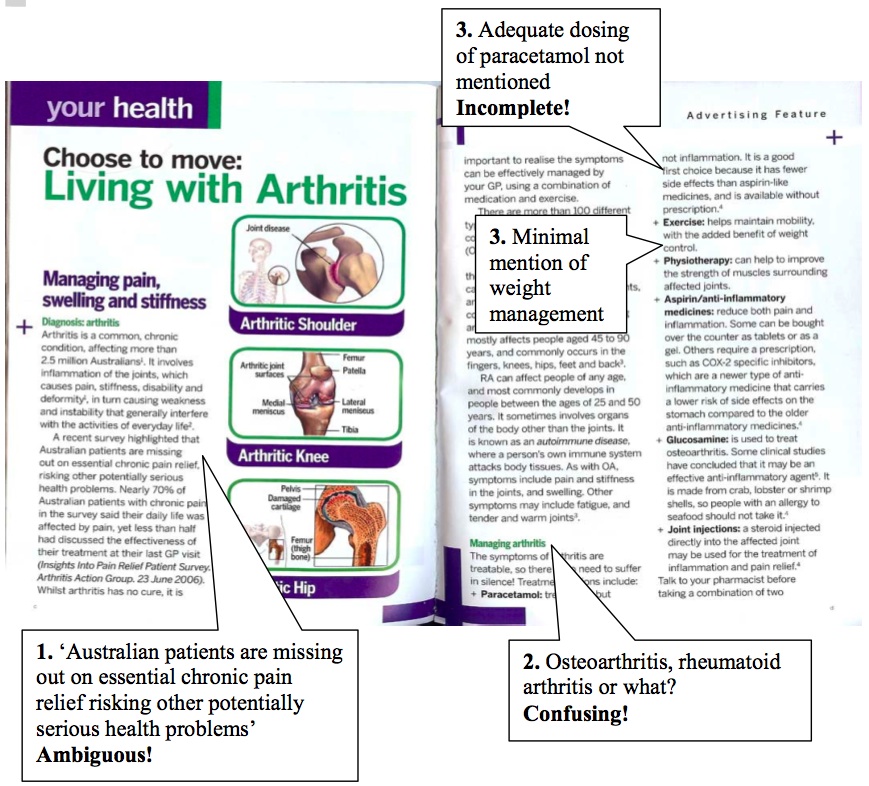

This advertorial is presented as consumer information on arthritis but hardly hides its main aim: half of the brochure promotes Cox-2 inhibitors, which include Pfizer’(tm)s Celebrex(r) (celecoxib).
1. The brochure states ‘A recent survey highlighted that Australian patients are missing out on essential chronic pain relief, risking other potentially serious health problems’. This sentence is ambiguous. It could be understood to claim that uncontrolled pain causes ‘serious health problems’. If so, what problems? Another understanding is a claim that patients are risking adverse effects because they are taking the wrong drugs. If so, which drugs? Whatever the interpretation, this may make readers feel that they are missing out on the best treatment so they should ask their doctor for a COX-2 inhibitor! We could not identify a published paper on the survey. However, the report of a larger study by the Arthritis Action Group acknowledges that the study received funding from Pfizer and its subsidiary Pharmacia.[1]
2. It is not clear which disease the brochure is about. While the advertorial describes the different types of arthritis and focuses in particular on the two most common types of arthritis, osteoarthritis and rheumatoid arthritis, the management section covers only osteoarthritis.
3. The brochure is incomplete. It fails to include weight management as a strategy to alleviate symptoms. Instead it merely mentions that exercise can have the ‘added benefit of weight control’. It does not mention that the inadequate dosing of paracetamol (incorrect dose or dosing interval) may prevent some patients from getting the full benefit from paracetamol.
4. The photo of these ‘arthritis sufferers’ was used extensively in Celebrex(r) promotional campaigns targeting doctors. This is a common approach used by drug companies to sidestep the ban on direct-to-consumer advertising (DTCA) of prescription medicines in Australia. The same illustration is used in the promotion to consumers and to prescribers. However, the name of the product advertised (in this case Celebrex(r)) is omitted in the consumer promotion to avoid blatant DTCA. If doctors are shown the brochure by patients, they cannot fail to recognise the product being promoted!
5. ‘One anti-inflammatory medicine was withdrawn from the market because of an increased risk of heart attack and stroke’. Pfizer omits to say that this anti-inflammatory medicine withdrawn from the market was a Cox-2 specific inhibitor, and that there is good scientific evidence to suggest that this is likely to be an effect of all Cox-2 specific inhibitors to varying extents.[2],[3]
6. ‘[Cox-2] inhibitors carry a much lower risk of gastrointestinal ulcers and bleeding’.
The reference for this statement is Arthritis Australia’s website. From the information given on this website, Arthritis Australia seems mainly or entirely funded by drug companies through the Medicines Australia Community Care for Arthritis Australia Program. In 2007, we do not know what the most appropriate NSAID is for a given patient because several important questions about their relative cardiovascular and gastrointestinal safety are still unanswered. However, it must be noted that ‘much lower risk’ has never been shown with celecoxib in proper randomised controlled
[3] trials.[4] Some Cox-2 selective drugs may be safer for the stomach but any benefit is unlikely to be large and may not last long. Furthermore, the sad story of rofecoxib has clearly shown that there was no point in saving your stomach if you lose your heart! Several recent studies have shown an increased cardiovascular risk with celecoxib and so it is important to consider the overall safety of this product.[5]
7. ‘Stomach problems can be avoided - speak to your doctor’. While some NSAIDs may be safer than others, none has been shown to be entirely risk-free.[6] To pretend the opposite is deceptive! The safest approach is to use paracetamol instead of NSAIDs.
8. Reference 6 is the Celebrex(r) Product Information, just in case the reader has not yet realized that this consumer information on ‘arthritis’ is a disguised advertorial for Celebrex(r)!
Confusing, misleading, incomplete, deceptive, this consumer advertorial by Pfizer is a very worrying example of de facto DTCA. Patients and the public need reliable information they can trust. Consumer information provided by drug companies aims to promote product sales first and is fundamentally biased. There is no role for industry in the provision of information about diseases or comparing treatments.
1. Woolf AD, Zeidler H, Haglund U, Carr AJ, Chaussade S, Cucinotta D, Veale DJ, Martin-Mola E. Musculoskeletal pain in Europe: its impact and a comparison of population and medical perceptions of treatment in eight European countries. Ann Rheum Dis. 2004 Apr;63(4):342-7.
2. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs. An update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007 Mar 27;115:1634-42.
3. Chen LC, Ashcroft DM. Risk of myocardial infarction associated with selective COX-2 inhibitors: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf. 2007 Apr 25; [Epub ahead of print].
4. Juni P, Rutjes A, Dieppe P. Are selective COX 2 inhibitors superior to traditional non steroidal anti-inflammatory drugs? BMJ. 2002 Jun 1;324: 1284-8.
5. Graham DJ. COX-2 inhibitors, other NSAIDs and cardiovascular risk. The seduction of common sense. JAMA. 2006 Oct 4;296:1653-6.
6. Chan FK, Wong VW, Suen BY, Wu JC, Ching JY et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding inpatients at very high risk: a double-blind, randomised trial. Lancet. 2007 May 12;369:1621-6.
Indexes:
AdWatch (Australia)
Page views since 15 March 2010: 16685
Comments
Our members can see and make comments on this page.


